Have you ever wondered what makes neon lights have such funky colors? Did you know that neon isn't the only color used in those colorful lights? Neon lights have been around since the first part of the 20th century, and from the 1920's-50's they were all the rage. Even though cheaper alternatives have been created since then, love of the original neon lights still have a following.
 |
| Example of an old neon sign. |
There is more than just neon used in the neon lights. It was discovered that if you had a tube of neon and ran a charge of electricity through it, it would glow with a red light. Only a little neon is needed, but you have to use a large amount of electric charge (even though the actual input is low, which makes them really energy efficient). Neon lights also don't have filaments, so they don't burn out. Instead, what typically happens is the metal on the ends degrades, but that takes a long time to happen (which makes neon lights last a long time). But scientists found out that neon wasn't the only gas that would glow like that.
Neon is a gas in the atmosphere and part of the periodic table of elements known as the noble gases. The noble gases are gases on the right side of the table that are unreactive to other elements. These are unreactive because they won't bond with other elements. Why won't they bond? Because the outermost ring of electrons in the atom has to be able to share electrons with other atoms. For example, 8 is the magic number of electrons. If there are 8 electrons, then that's what the atom wants. But if your atom has an outer ring of 7 electrons, then it most wants to share an electron with an atom that only has 1 electron in its outermost ring. A ring with 6 electrons wants either an atom with 2 electrons in its outermost ring or two atoms with 1 electron in their outermost rings. Make sense? Well, the noble gases are called "noble" after the royalty of the old days: they didn't mix with others. So there weren't really any practical uses for the noble gases, until the electricity through them was found out.
There are six naturally occurring noble gases: helium, neon, argon, krypton, xenon, and radon. Helium is only one of these elements that does not have eight electrons in its outermost electron ring (it has only two electrons in the entire atom). Radon is not used in neon lights because it is radioactive. Helium, argon, krypton, and xenon are all used in addition to neon in these style of lights, with each one glowing a specific color.

Neon glows with a bright red color.

Helium glows with a fainter reddish-pink color (to make it brighter, sometimes a small amount of mercury is added).
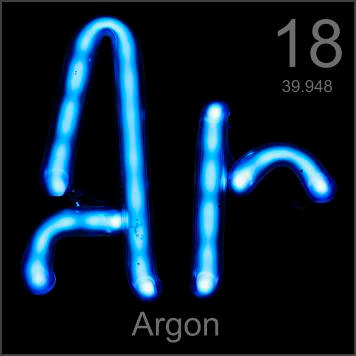
Argon glows with a light blue color.
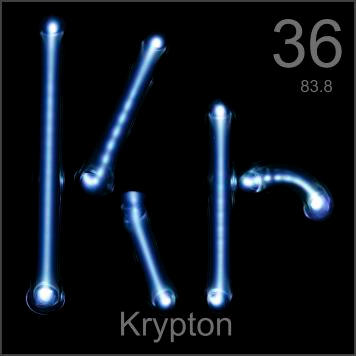
Krypton glows with a yellowish-white color, which can be used with colored glass to make all sorts or colors.

Xenon glows with a bright purple color.
The combinations of helium, neon, argon, krypton, and xenon along with other elements like mercury and carbon dioxide can produce many different colors. However, a lot of tubes are also colored in a variety of colors using phosphors, which are substances that can glow (think of phosphorus). When you have a gas that gives off a certain color, and you can put a color of tube around that gas, then creating just about any color is as simple as when you mix paint. For example, if you have the blue argon light, and put a yellow tube, then you will get a green light.
Today we had a trip through chemistry, but wasn't it interesting?



